An Investigation on Structural and Optical Properties of Zn1−xMgxS Thin Films Deposited by RF Magnetron Co-Sputtering Technique
Abstract
:1. Introduction
2. Materials and Methods
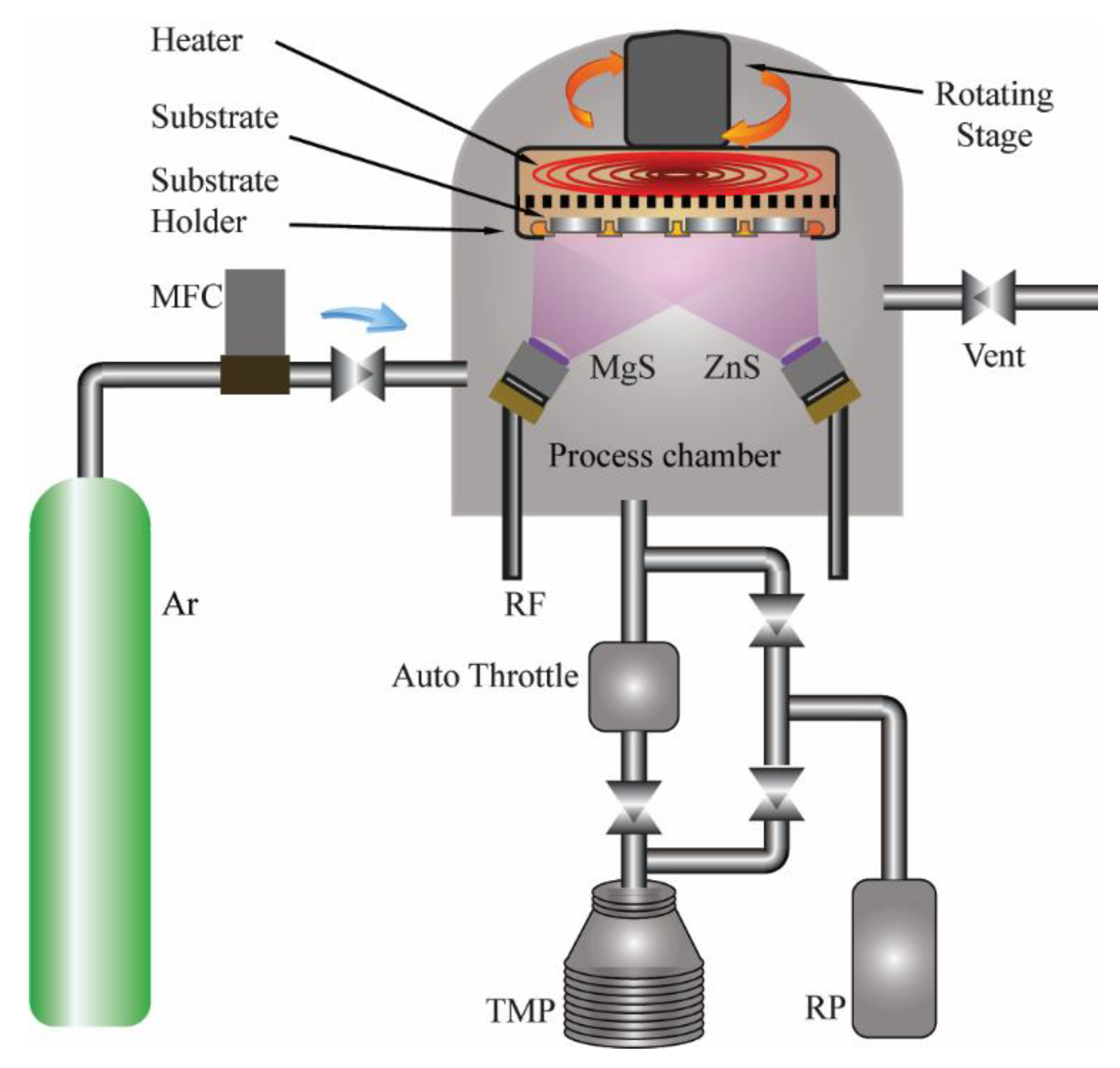
2.1. Deposition of Thin Films
2.2. Characterization of Thin Films
3. Results and Discussion
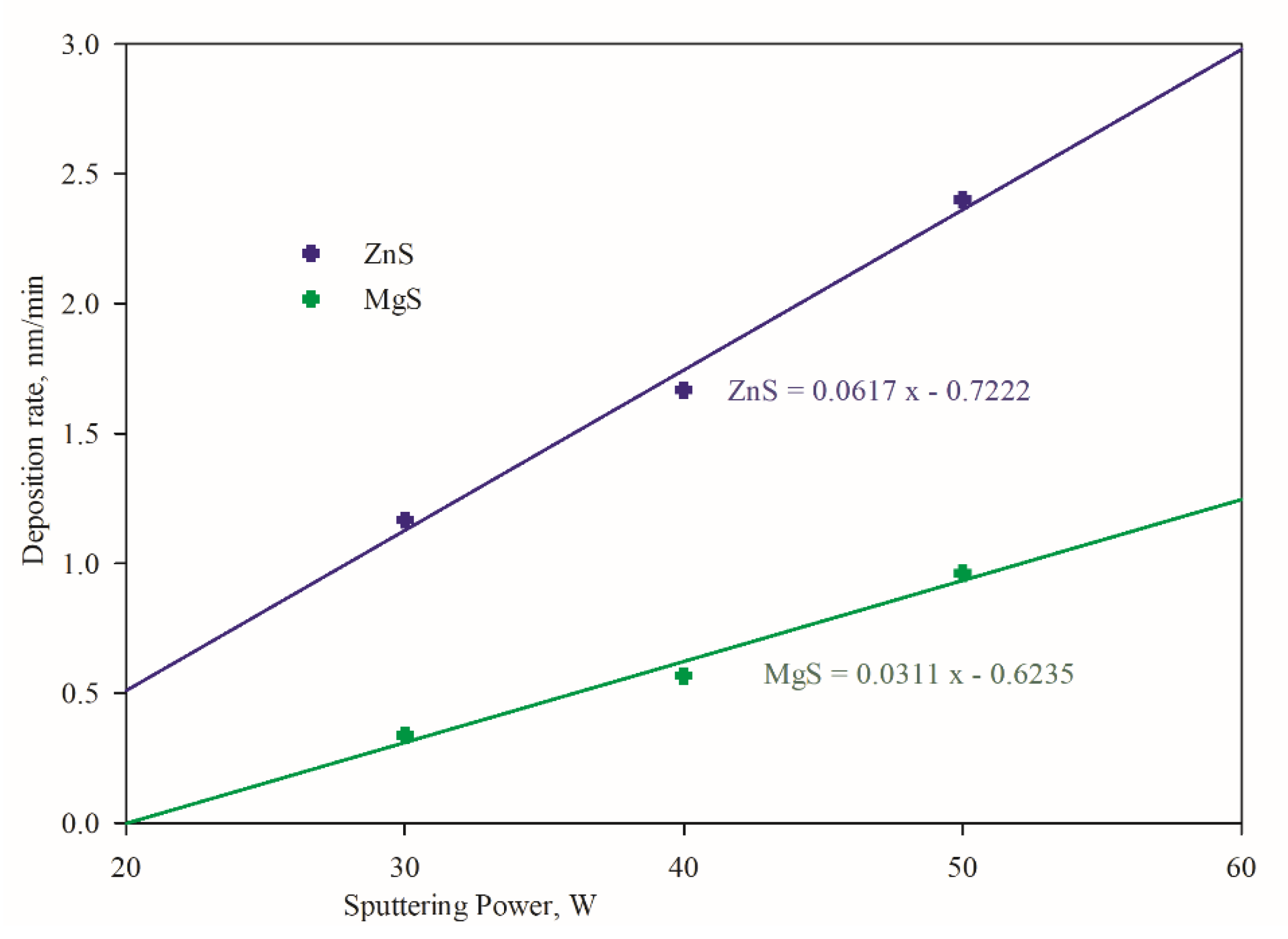
3.1. Compositional Variations
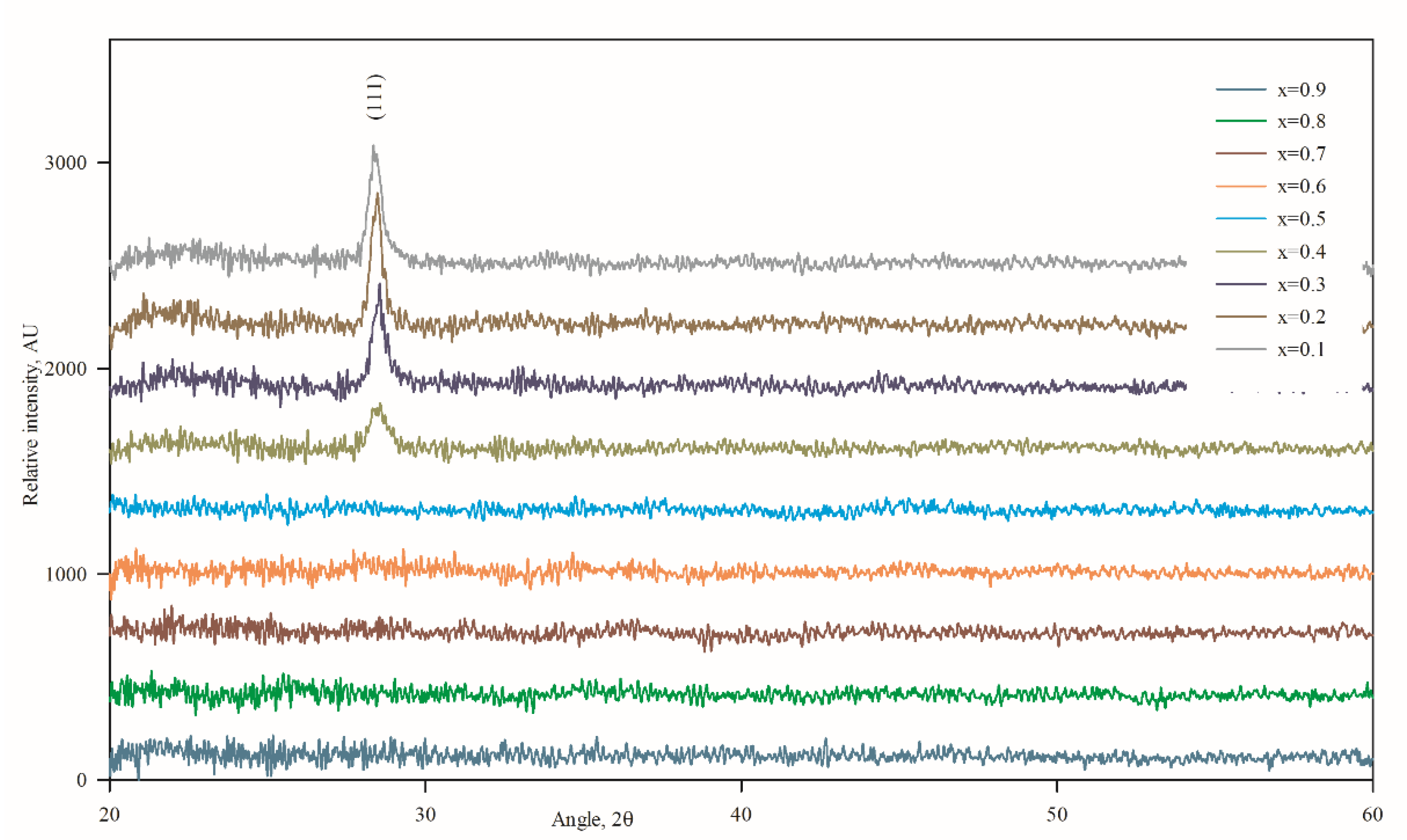
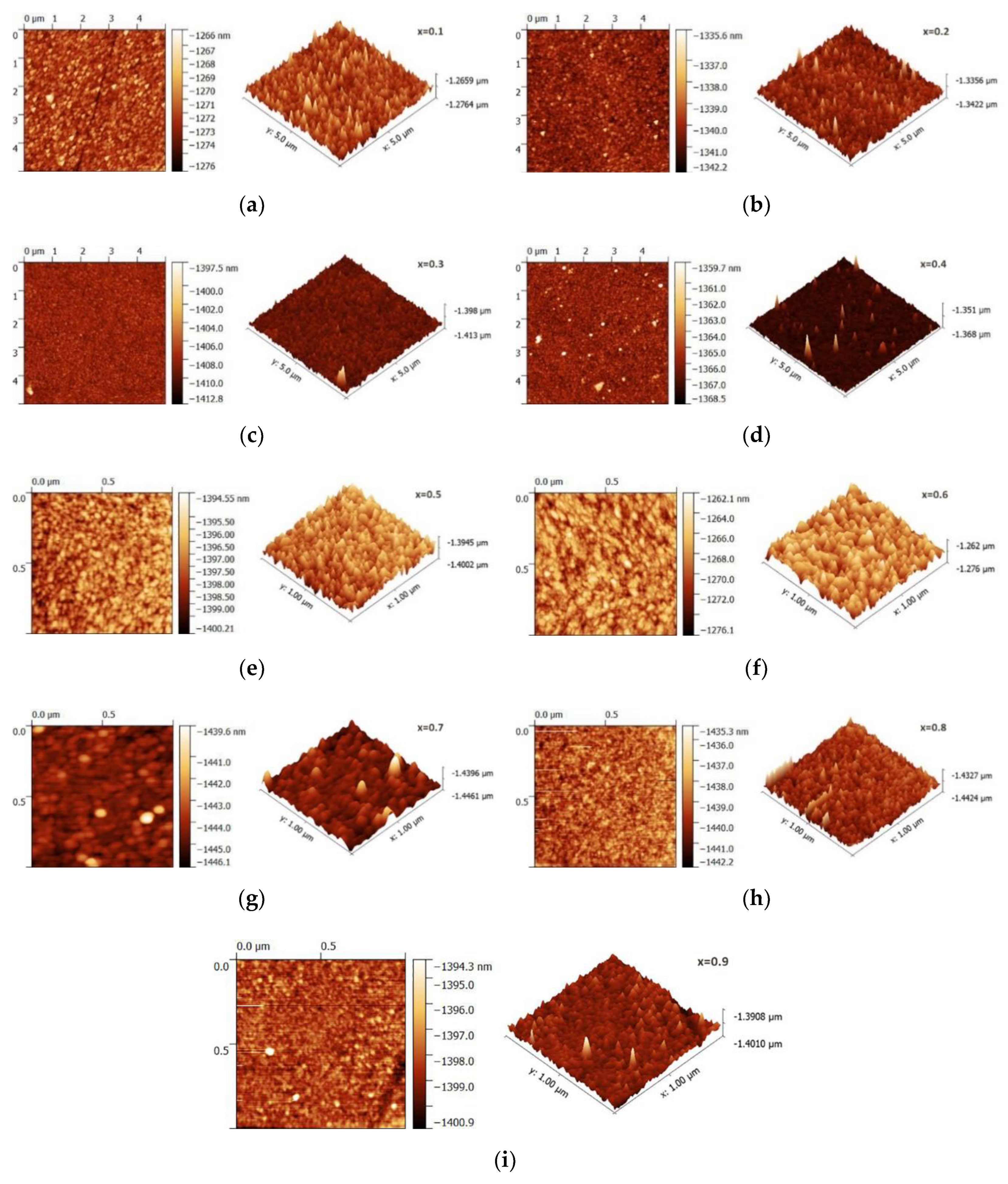
3.2. Structural Properties
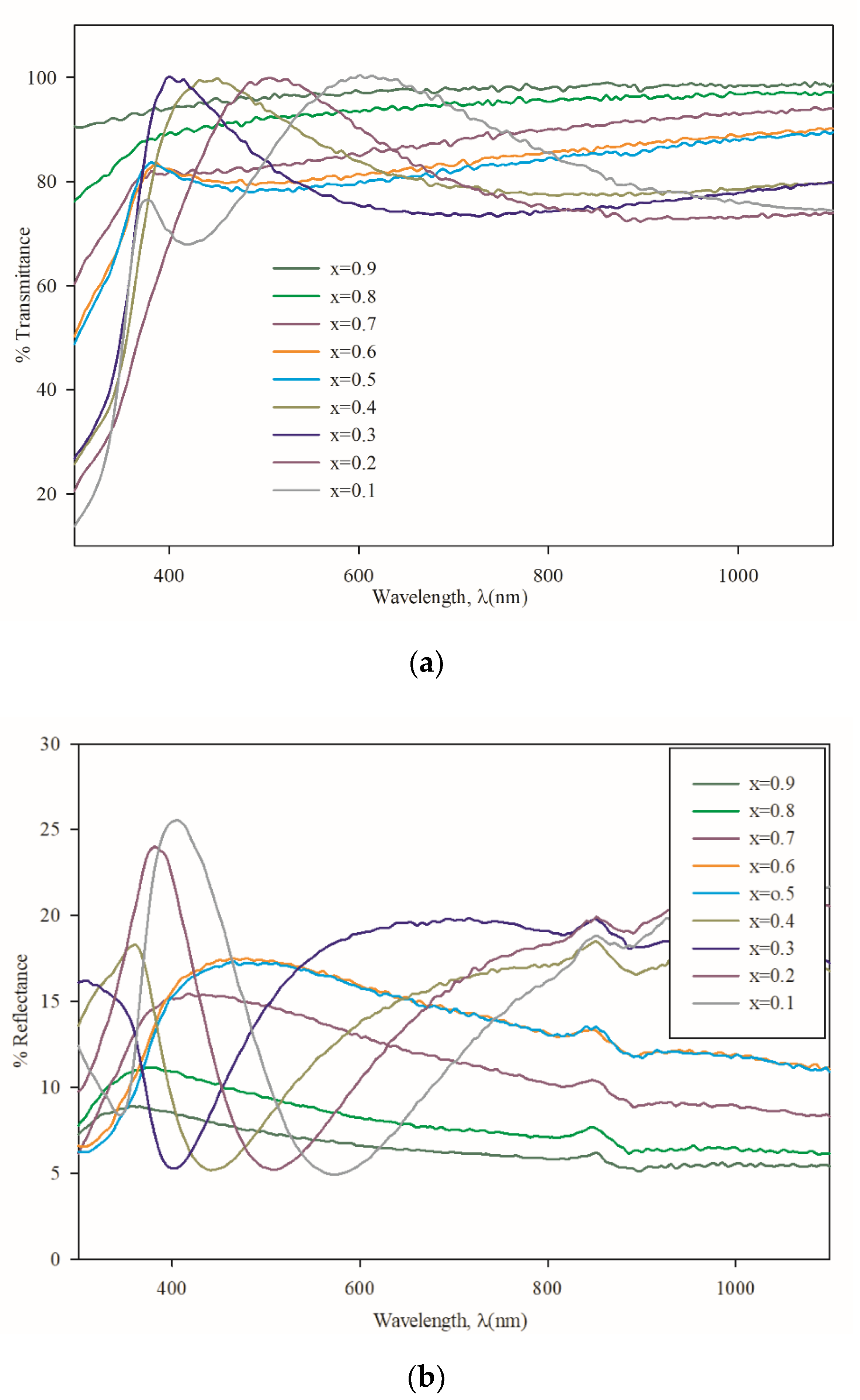
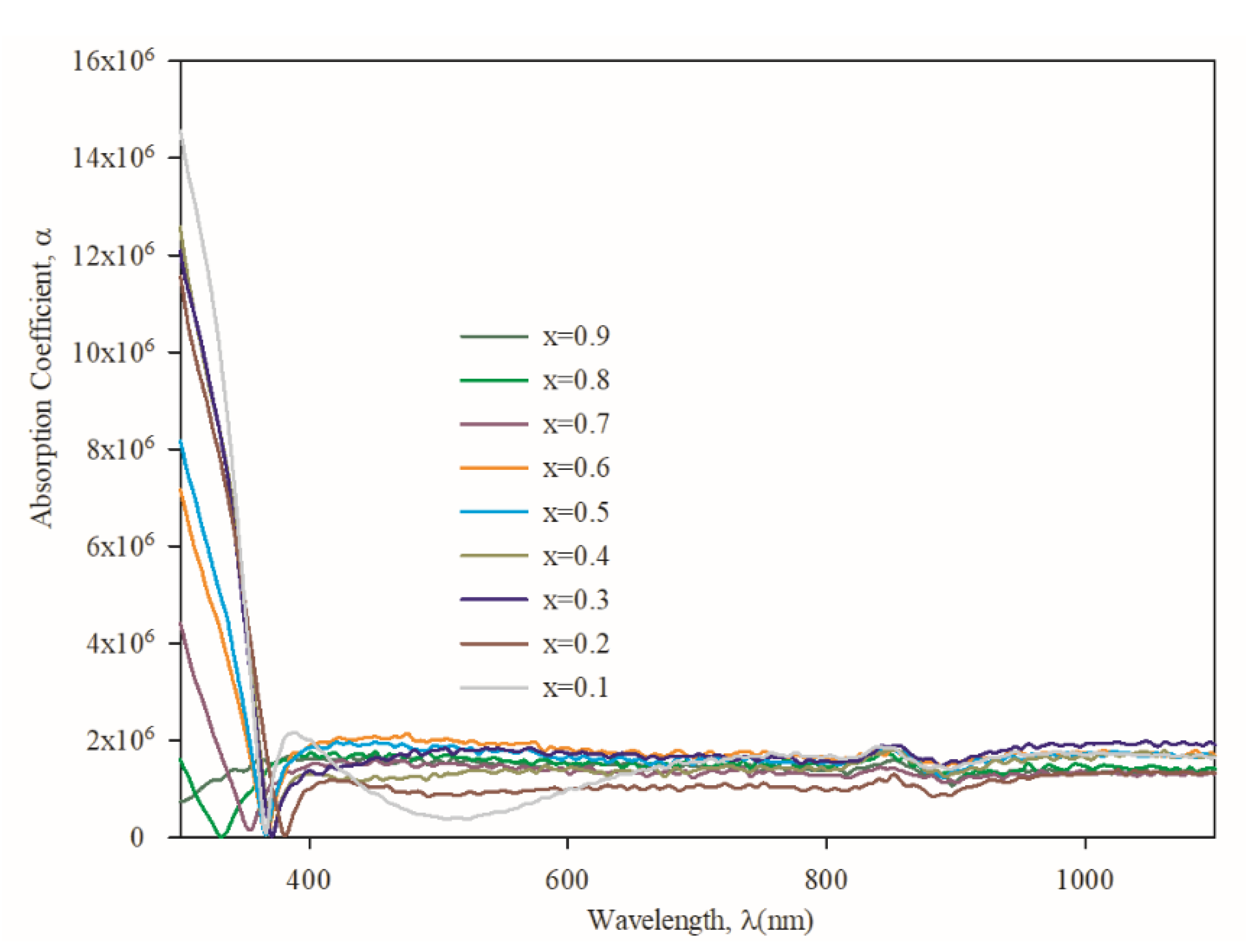
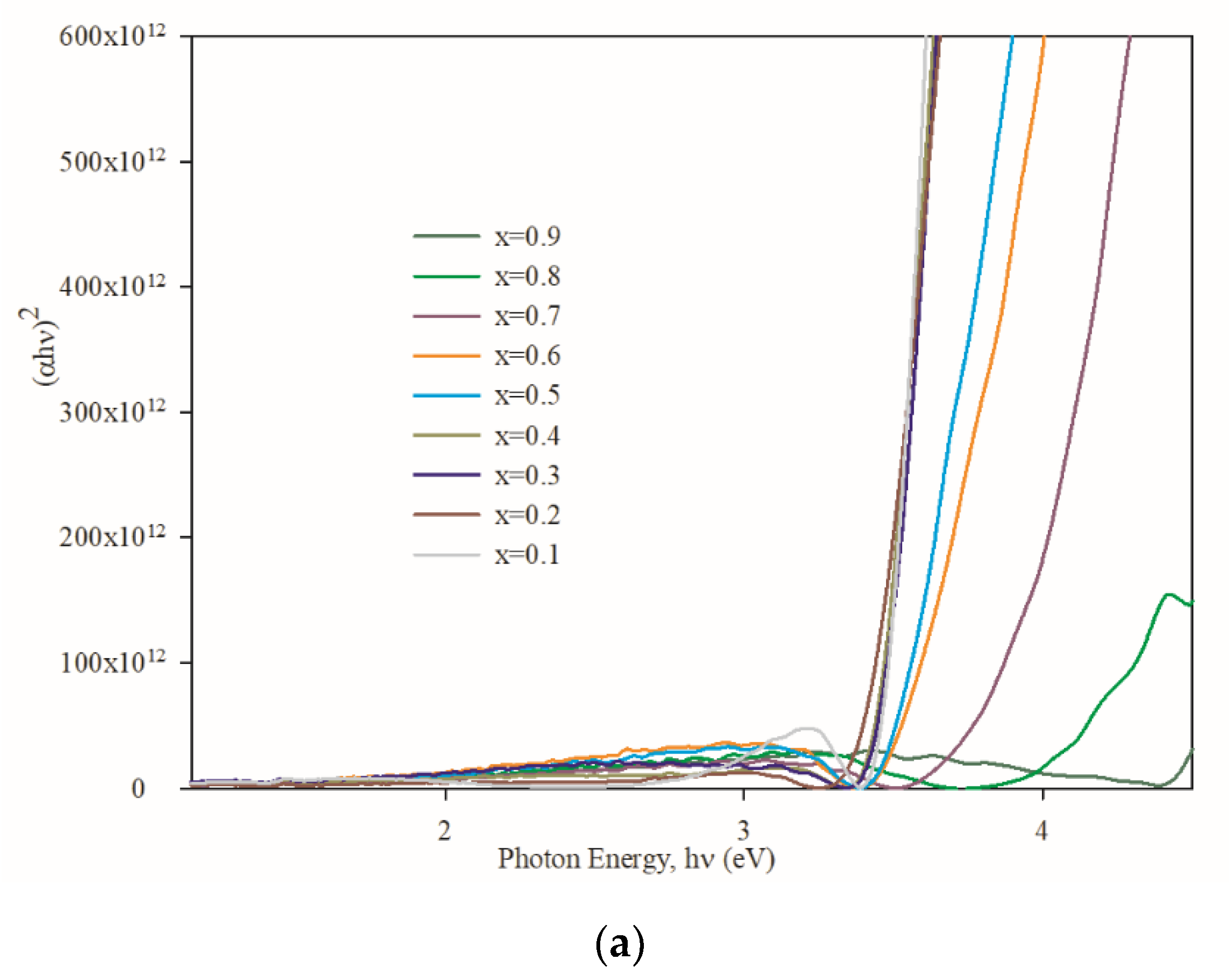
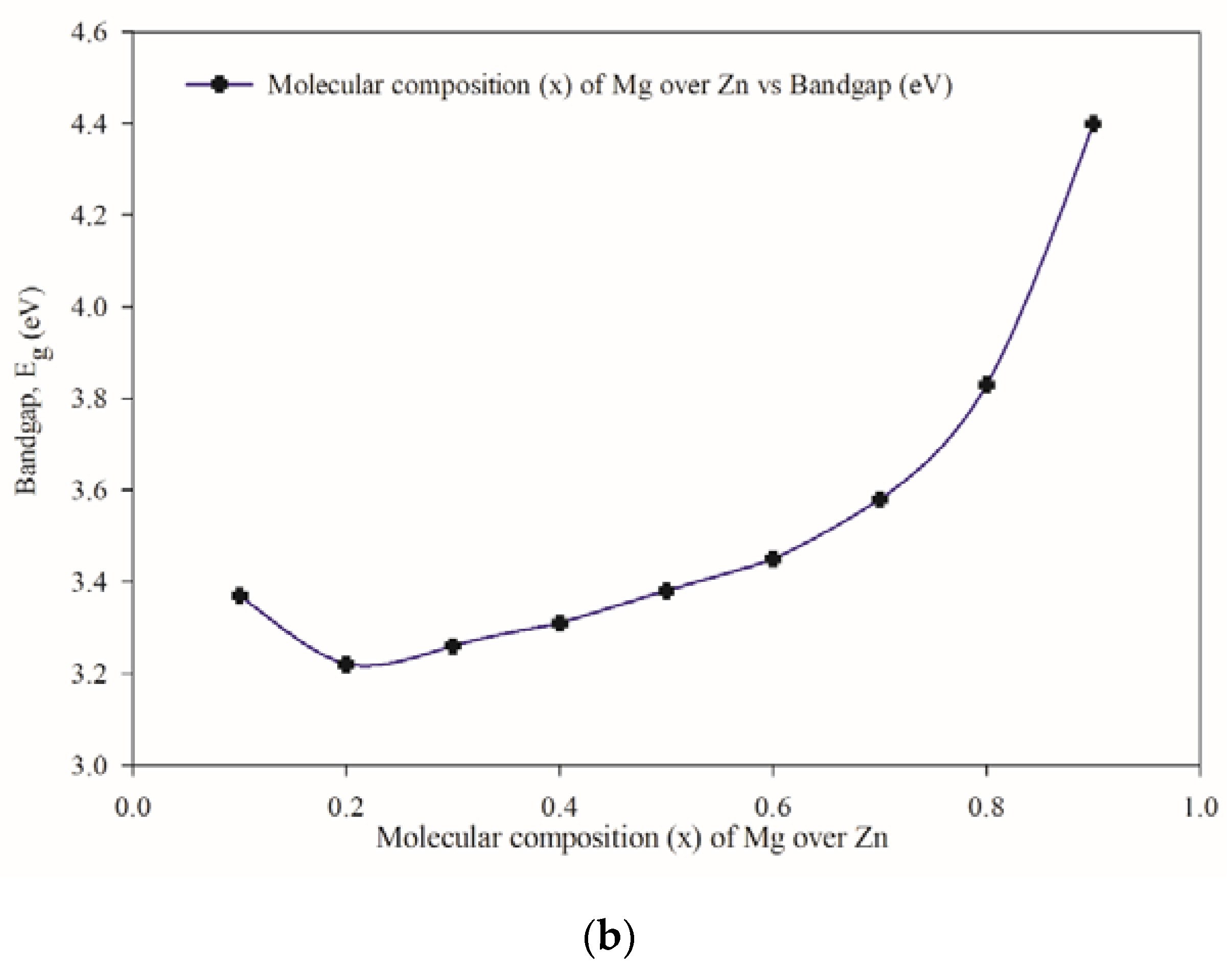
3.3. Optical Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Islam, M.A.; Hossain, M.S.; Aliyu, M.M.; Husna, J.; Huda, Q.U.; Karinm, M.R.; Sopian, K.B.; Amin, N. Growth of Wide Bandgap CdS Thin Films as Window Layers of Ultra Thin CdTe Solar Cells. In Proceedings of the 22nd International Photovoltaic Science and Engineering Conference, Hangzhou, China, 5–9 November 2012; pp. 1–5. [Google Scholar]
- Matin, R.; Bashar, M.S.; Sultana, M.; Ahmed, A.N.; Gafur, A. Annealing effects on the structural, optical and electrical properties of chemically deposited CdS thin films using NH4Cl complexing agent. Int. J. Nanoelectron. Mater. 2018, 11, 221–232. [Google Scholar]
- Matin, M.A.; Amin, N.; Zaharim, A.; Sopian, K. Ultra thin high efficiency CdS/CdTe thin film solar cells from numerical analysis. In Proceedings of the 8th WSEAS International Conference on NON-Linear Analysis, Non-Linear Systems and Chaos, La Laguna, Canary Islands, Spain, 1–3 July 2009; pp. 338–344. [Google Scholar]
- Rupom, R.H.; Matin, R.; Bashar, M.S.; Sultana, M.; Rahaman, M.; Gafur, M.A.; Hakim, M.; Hossain, M.K.; Bhuiyan, M.M.R.; Ahmed, F. Fabrication and Characterization of Cadmium Sulfide (CdS) Buffer Layer Thin Film by Using Chemical Bath Deposition Method for Solar Cell. Am. Int. J. Res. Sci. 2016, 14, 10–15. [Google Scholar]
- Majeed, S.; Siraj, K.; Naseem, S.; Khan, M.F.; Irshad, M.; Faiz, H.; Mahmood, A. Structural and optical properties of gold-incorporated diamond-like carbon thin films deposited by RF magnetron sputtering. Mater. Res. Express 2017, 4, 076403. [Google Scholar] [CrossRef]
- Haque, F.; Rahman, K.S.; Islam, M.A.; Rashid, M.J.; Akhtaruzzaman, M.; Alam, M.M.; Alothman, Z.A.; Sopian, K.B.; Amin, N. Growth optimization of ZnS thin films by RF magnetron sputtering as prospective buffer layer in thin film solar cells. Chalcogenide Lett. 2014, 11, 189–197. [Google Scholar]
- Islam, M.A.; Hossain, M.S.; Aliyu, M.M.; Sulaiman, Y.; Karim, M.R.; Sopian, K.; Amin, N. Comparative study of ZnS thin films grown by chemical bath deposition and magnetron sputtering. In Proceedings of the 2012 7th International Conference on Electrical and Computer Engineering, Dhaka, Bangladesh, 20–22 December 2012; pp. 86–89. [Google Scholar]
- Hossain, M.; Rahman, K.; Islam, M.; Akhtaruzzaman, M.; Misran, H.; Alghoul, M.; Amin, N. Growth optimization of ZnxCd1-xS films on ITO and FTO coated glass for alternative buffer application in CdTe thin film solar cells. Opt. Mater. 2018, 86, 270–277. [Google Scholar] [CrossRef]
- Hossain, S.; Kabir, H.; Rahman, M.M.; Hasan, K.; Bashar, M.S.; Rahman, M.; Gafur, A.; Islam, S.; Amri, A.; Jiang, Z.-T.; et al. Understanding the shrinkage of optical absorption edges of nanostructured Cd-Zn sulphide films for photothermal applications. Appl. Surf. Sci. 2017, 392, 854–862. [Google Scholar] [CrossRef]
- Hossain, M.S.; Rahman, K.S.; Karim, M.R.; Aijaz, M.O.; Dar, M.A.; Shar, M.A.; Misran, H.; Amin, N. Impact of CdTe thin film thickness in ZnxCd1−xS/CdTe solar cell by RF sputtering. Sol. Energy 2019, 180, 559–566. [Google Scholar] [CrossRef]
- Akh, S.; Matin, R.; Bashar, M.S.; Kowsar, A.; Rahaman, M.; Mahmood, Z.H.; Akhanda, S. Experimental Study on Structural, Optical and Electrical Properties of Chemical Bath Deposited CdZnS Thin Films. J. Fundam. Renew. Energy Appl. 2017, 7. [Google Scholar] [CrossRef] [Green Version]
- Inoue, R.; Kitagawa, M.; Nishigaki, T.; Ichino, K.; Kobayashi, H.; Ohishi, M.; Saito, H. Optical band gap of ZnxMg1−xS thin films with composition x between 0.14 and 1. J. Cryst. Growth 1998, 184, 1076–1080. [Google Scholar] [CrossRef]
- Khan, I.; Ahmad, I.; Aliabad, H.A.R.; Maqbool, W. Effect of phase transition on the optoelectronic properties of Zn1−xMgxS. J. Appl. Phys. 2012, 112, 73104. [Google Scholar] [CrossRef]
- Kimijima, H.; Kitagawa, M.; Inoue, R.; Shiraishi, N.; Hoashi, M.; Ichino, K.; Kobayashi, H. Deposition and characterization of ZnxMg1−xS thin films on amorphous substrates. Appl. Surf. Sci. 1997, 113, 432–435. [Google Scholar] [CrossRef]
- Kalpana, G.; Palanivel, B.; Thomas, R.M.; Rajagopalan, M. Electronic and structural properties of MgS and MgSe. Phys. B Condens. Matter 1996, 222, 223–228. [Google Scholar] [CrossRef]
- Okuyama, H.; Kishita, Y.; Ishibashi, A. Quaternary alloy Zn1−xMgxSySe1−y. Phys. Rev. B 1998, 57, 2257–2263. [Google Scholar] [CrossRef]
- Bradford, C.; Moug, R.; Curran, A.; Thuau, D.; Warburton, R.J.; Prior, K. Growth and Characterization of ZnMgS and ZnMgS/ZnSe Quantum Wells grown on GaAs (100) by Using MBE. J. Korean Phys. Soc. 2008, 53, 3000–3003. [Google Scholar] [CrossRef]
- Sou, I.K.; Wu, M.C.W.; Sun, T.; Wong, K.S.; Wong, G.K.L. MBE-Grown ZnMgS ultra-violet photodetectors. J. Electron. Mater. 2001, 30, 673–676. [Google Scholar] [CrossRef]
- Rodriguez, A.; Shattuck, J.; Zhang, X.; Li, P.; Parent, D.; Ayers, J.; Jain, F. Photo-Assisted MOVPE Growth of ZnMgS on (100) Si. MRS Proc. 2001, 692, 625–630. [Google Scholar] [CrossRef] [Green Version]
- Brightwell, J.W.; Ray, B.; White, S. Phase structure of Zn1-xMgxS prepared by reaction of mixed chlorides with H2S. J. Mater. Sci. Lett. 1984, 3, 951–954. [Google Scholar] [CrossRef]
- Amah, A.N. Synthesis and optical characterization of ZnMgS:Eu nanoparticles. Turk. J. Phys. 2013, 37, 289–295. [Google Scholar] [CrossRef]
- Dive, A.; Gattu, K.; Huse, N.; Upadhayay, D.R.; Phase, D.; Sharma, R.B. Single step chemical growth of ZnMgS nanorod thin film and its DFT study. Mater. Sci. Eng. B 2018, 228, 91–95. [Google Scholar] [CrossRef]
- Dong, L.M.; Li, M.J.; Liu, X.D.; Wu, K.J.; Guo, Y.K. Study on structural and luminescence properties of ZnS: Mg2+ quantum dots synthesized with aqueous method. J. Ovonic Res. 2016, 12, 155–161. [Google Scholar]
- Shahid, M.Y.; Asghar, M.; Zafar, M.; Ilyas, S.Z.; Arbi, H.M. Role of magnesium in ZnS structure: Experimental and theoretical investigation. AIP Adv. 2016, 6, 025019. [Google Scholar] [CrossRef] [Green Version]
- Shao, L.-X.; Chang, K.-H.; Hwang, H.-L. Zinc sulfide thin films deposited by RF reactive sputtering for photovoltaic applications. Appl. Surf. Sci. 2003, 212, 305–310. [Google Scholar] [CrossRef]
- Warren, B.E.; Muldawer, L. X-Ray Diffraction. Phys. Today 1970, 23, 53. [Google Scholar] [CrossRef]
- Morinaga, Y.; Okuyama, H.; Akimoto, K. Photopumped Blue Lasers with ZnSSe-ZnMgSSe Double Heterostructure and Attempt at Doping in ZnMgSSe. Jpn. J. Appl. Phys. 1993, 32, 678–680. [Google Scholar] [CrossRef]
- Guo, Y.-D.; Yang, Z.-J.; Gao, Q.-H.; Dai, W. Structural and elastic properties of MgS via first-principle calculations. Phys. B Condens. Matter 2008, 403, 2367–2371. [Google Scholar] [CrossRef]
- Hotje, U.; Rose, C.; Binnewies, M. Lattice constants and molar volume in the system ZnS, ZnSe, CdS, CdSe. Solid State Sci. 2003, 5, 1259–1262. [Google Scholar] [CrossRef]
- Lucero, M.J.; Henderson, T.M.; E Scuseria, G. Improved semiconductor lattice parameters and band gaps from a middle-range screened hybrid exchange functional. J. Phys. Condens. Matter 2012, 24, 145504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qadri, S.; Skelton, E.F.; Hsu, D.; Dinsmore, A.D.; Yang, J.; Gray, H.F.; Ratna, B.R. Size-induced transition-temperature reduction in nanoparticles of ZnS. Phys. Rev. B 1999, 60, 9191–9193. [Google Scholar] [CrossRef]
- Drief, F.; Tadjer, A.; Mesri, D.; Aourag, H. First principles study of structural, electronic, elastic and optical properties of MgS, MgSe and MgTe. Catal. Today 2004, 89, 343–355. [Google Scholar] [CrossRef]
- Yuan, P.F.; Ding, Z. Ab initio calculation of elastic properties of rock-salt and zinc-blend MgS under pressure. Phys. B Condens. Matter 2008, 403, 1996–1999. [Google Scholar] [CrossRef]
- Lee, S.G.; Chang, K.J. First-principles study of the structural properties of MgS-, MgSe-, ZnS-, and ZnSe-based superlattices. Phys. Rev. B 1995, 52, 1918–1925. [Google Scholar] [CrossRef] [PubMed]
- Othman, Z.J.; Ayed, S.; Matoussi, A.; Khemakhem, H. Optical and Raman studies of Zn1-xMgxO ceramic pellets. Vib. Spectrosc. 2016, 85, 208–214. [Google Scholar] [CrossRef]
- Phillips, J.C.; Harrison, W.A. Bonds and Bands in Semiconductors. Phys. Today 1974, 27, 67. [Google Scholar] [CrossRef]
- Fewster, P.; Birkholz, M. Thin Film Analysis by X-ray Scattering; Wiley-VCH Verlag GmbH & Co. KGaA: Berlin, Germany, 2006. [Google Scholar]
- Pankove, J.I. Optical Processes in Semiconductors, 2nd ed.; Dover Publications, Inc.: Mineola, NY, USA, 2010. [Google Scholar]
- Asano, T.; Funato, K.; Nakamura, F.; Ishibashi, A. Epitaxial growth of ZnMgTe and double heterostructure of on GaAs substrate by metalorganic chemical vapor deposition. J. Cryst. Growth 1995, 156, 373–376. [Google Scholar] [CrossRef]
- Nashiki, H.; Suemune, I.; Kumano, H.; Suzuki, H.; Obinata, T.; Uesugi, K.; Nakahara, J. Excitonic luminescence up to room temperature in a ZnSe/MgS superlattice. Appl. Phys. Lett. 1997, 70, 2350–2352. [Google Scholar] [CrossRef]
- O’Neil, M.; Marohn, J.; McLendon, G. Dynamics of electron-hole pair recombination in semiconductor clusters. J. Phys. Chem. 1990, 94, 4356–4363. [Google Scholar] [CrossRef]
- Spanhel, L.; Haase, M.; Weller, H.; Henglein, A. Surface modification and stability of strong luminescing CdS particles. J. Am. Chem. Soc. 1987, 109, 5649–5655. [Google Scholar] [CrossRef]
- Kruse, C.; Alexe, G.; Klude, M.; Heinke, H.; Hommel, D. High Reflectivity p-Type Doped Distributed Bragg Reflectors Using ZnSe/MgS Superlattices. Phys. Status Solidi 2002, 229, 111–115. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Liang, C.; Wang, G.; Peng, X. Catalytic growth and photoluminescence properties of semiconductor single-crystal ZnS nanowires. Chem. Phys. Lett. 2002, 357, 314–318. [Google Scholar] [CrossRef]
- Camacho, G.J.O.; Marín, D.M.; Andrada, R.T.; Loscos, M.J.C.; Mariño, I.M.; Mariño, M.A.M. Efectos de la ingestión de cafeína sobre el rendimiento, la peroxidación lipídica y las vitaminas A, E y C, en sujetos sometidos a una prueba de esfuerzo máxima. Arch. Med. Deport 2002, 19, 371–375. [Google Scholar]


| Deposited Film | EDS: Atomic % | |
|---|---|---|
| ZnS | Zn | 80.16 |
| S | 19.84 | |
| MgS | Mg | 97.85 |
| S | 2.15 | |
| Power (W) | EDS: Atomic % | “x” Value | Thickness (nm) | |||
|---|---|---|---|---|---|---|
| MgS | ZnS | Zn | Mg | S | ||
| 40.00 | 20.00 | 8.26 | 79.81 | 11.94 | 0.91 | 72.00 ± 0.54 |
| 40.00 | 26.00 | 15.18 | 65.24 | 19.58 | 0.81 | 69.00 ± 0.83 |
| 39.28 | 33.00 | 25.97 | 57.66 | 16.37 | 0.69 | 86.00 ± 0.56 |
| 33.28 | 36.24 | 30.42 | 39.72 | 29.87 | 0.57 | 77.00 ± 0.64 |
| 32.06 | 41.41 | 42.66 | 44.50 | 12.84 | 0.51 | 72.00 ± 1.00 |
| 29.65 | 45.37 | 39.37 | 27.89 | 32.74 | 0.41 | 85.00 ± 0.21 |
| 29.65 | 65.16 | 45.85 | 17.50 | 36.65 | 0.28 | 80.00 ± 0.64 |
| 25.24 | 67.34 | 50.00 | 13.97 | 36.04 | 0.22 | 90.00 ± 0.15 |
| 20.98 | 70.51 | 58.20 | 7.16 | 34.64 | 0.11 | 89.00 ± 0.79 |
| Molar Fraction (x) | Roughness (nm) | Grain Size (nm) |
|---|---|---|
| 0.9 | 0.571 | 28 |
| 0.8 | 0.523 | 31 |
| 0.7 | 0.546 | 35 |
| 0.6 | 0.516 | 43 |
| 0.5 | 0.527 | 50 |
| 0.4 | 0.492 | 73 |
| 0.3 | 0.623 | 103 |
| 0.2 | 0.414 | 186 |
| 0.1 | 0.839 | 144 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bashar, M.S.; Yusoff, Y.; Abdullah, S.F.; Rahaman, M.; Chelvanathan, P.; Gafur, A.; Ahmed, F.; Akhtaruzzaman, M.; Amin, N. An Investigation on Structural and Optical Properties of Zn1−xMgxS Thin Films Deposited by RF Magnetron Co-Sputtering Technique. Coatings 2020, 10, 766. https://doi.org/10.3390/coatings10080766
Bashar MS, Yusoff Y, Abdullah SF, Rahaman M, Chelvanathan P, Gafur A, Ahmed F, Akhtaruzzaman M, Amin N. An Investigation on Structural and Optical Properties of Zn1−xMgxS Thin Films Deposited by RF Magnetron Co-Sputtering Technique. Coatings. 2020; 10(8):766. https://doi.org/10.3390/coatings10080766
Chicago/Turabian StyleBashar, Muhammad Shahriar, Yulisa Yusoff, Siti Fazlili Abdullah, Mashudur Rahaman, Puvaneswaran Chelvanathan, Abdul Gafur, Farid Ahmed, Md Akhtaruzzaman, and Nowshad Amin. 2020. "An Investigation on Structural and Optical Properties of Zn1−xMgxS Thin Films Deposited by RF Magnetron Co-Sputtering Technique" Coatings 10, no. 8: 766. https://doi.org/10.3390/coatings10080766
APA StyleBashar, M. S., Yusoff, Y., Abdullah, S. F., Rahaman, M., Chelvanathan, P., Gafur, A., Ahmed, F., Akhtaruzzaman, M., & Amin, N. (2020). An Investigation on Structural and Optical Properties of Zn1−xMgxS Thin Films Deposited by RF Magnetron Co-Sputtering Technique. Coatings, 10(8), 766. https://doi.org/10.3390/coatings10080766









